In depth breakdown of boilingUpdated a month ago
Aim of boiling
Processes that take place during wort boiling:
- Wort sterilisation
- Enzyme inactivation
- Coagulation of proteins (hot break formation)
- Evaporation of water
- Removal of undesired volatiles
- Formation of aromatic and colouring compounds
- Ingredient additions
- Hop: Extraction and isomerization of hop components (see page 'Ingredients in boiling' for more information)
Wort sterilisation
Wort boiling (100°C at sea level) will kill spoilage bacteria such Lactobacillus and Pediococcus and wild yeast such as Brettanomyces by heat. Moreover, low pH and antibacterial activity of hop-derived compounds inhibit the growth of bacteria. This will prevent acid production, haze formation and off-flavour production by spoilage bacteria.
Enzyme inactivation
Most of the enzymes are inactivated during mash-out, but the remaining enzymes will be inactivated during boiling. Unfermentable sugars will not continue to be converted to fermentable sugars.
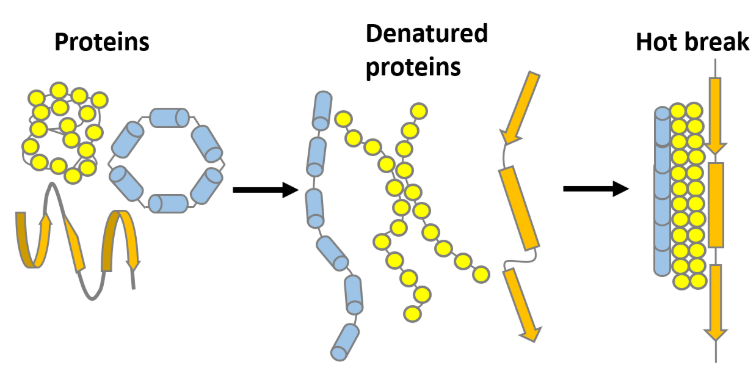
Hot break formation
Proteins (from malt) and polyphenols (from grain husks and hop) that are insoluble in hot wort precipitate as hot break. Proteins lose their structure due to denaturation by heat, which makes them insoluble in hot wort and makes them stick together as hot break.
The coagulation of proteins increases with longer boiling times, more vigorous movement of the boiling wort and a low pH. Insufficient coagulation and removal results in poor fermentation, due to adsorption of hot break to the yeast cell walls. Moreover, proteins and polyphenols can cause haze when present in excessive amounts.
Evaporation of water
Around 2.5% of the initial wort volume is evaporated during a 60 min boil (compared to 8-12% during conventional atmospheric boiling in breweries). Pre-boil gravity in the MiniBrew is usually 3-4 points lower than post-boil gravity. The evaporated water leaves the MiniBrew via the condensor or condenses into the mash-tun.
Removal of undesired volatiles
With the evaporation of water, unwanted volatiles like dimethyl sulphide (DMS) are also evaporated. DMS is an off-flavour with a cooked vegetable, sweet corn flavour. DMS is formed from S-methyl-methionine (SMM) by heat, SMM is present in high concentrations in pale malts (Pilsner, Pale Ale). DMS is volatile and most of it is lost by evaporation during boiling. Therefore, sufficient evaporation during wort boiling is important.
Formation of aromatic and colouring compounds
The Maillard reaction is a chemical reaction between amino acids and reducing sugars (e.g. lactose, maltose) followed by a cascade of reactions. Aroma and colour compounds like melanoidins (high molecular weight brown polymers) are formed during this reaction.